PCR, invented by Kary Mullis in 1983, revolutionized biotechnology by creating millions of DNA copies from a single sample. It allowed scientists to analyze genetic material from as small as a cell, a hair strand, or a blood drop, fostering groundbreaking research and diagnostics worldwide. Leading organizations like Countable Labs utilize advanced molecular approaches, pushing the boundaries of what PCR can achieve. PCR, a core technology for selectively multiplying and studying DNA fragments, is crucial for early disease detection, tracing organism genetic lineages, and preserving ecological diversity, impacting clinicians, researchers, and policymakers globally.
How PCR Works
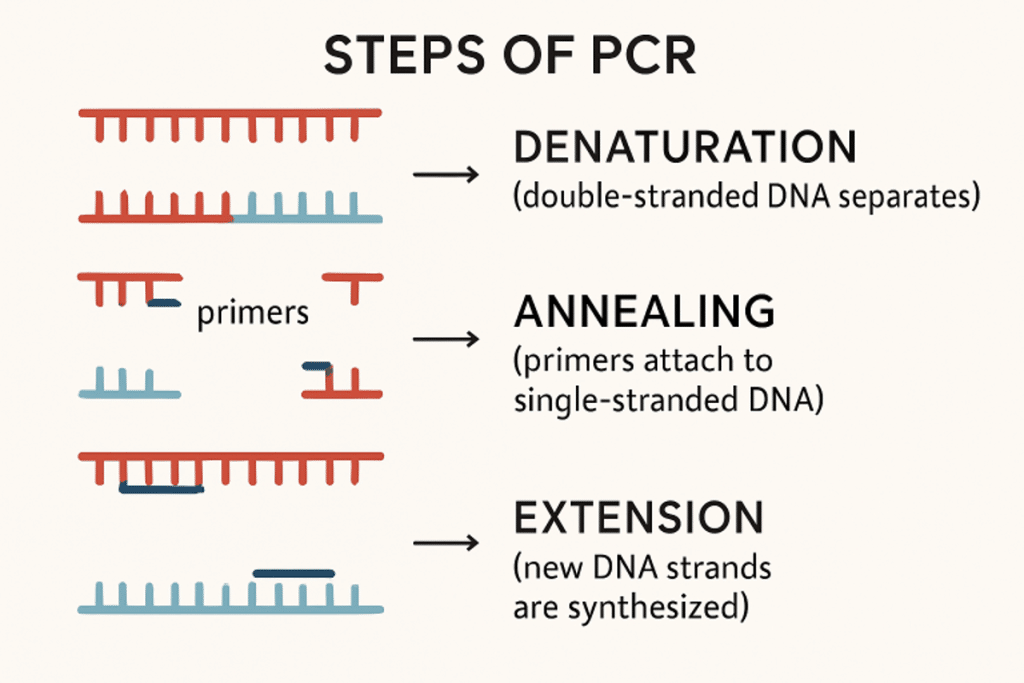
PCR’s elegance lies in its simplicity and efficiency, utilizing a rapid series of temperature changes to precisely copy a chosen segment of DNA. This intricate process, repeated multiple times, exponentially magnifies the initial DNA present, no matter how minuscule. The PCR cycle typically includes three critical stages, each guided by precise temperature control and essential biomolecular tools:
- Denaturation: At this phase, the DNA template solution is rapidly heated to a high temperature, generally between 94-98°C. This causes the normally stable double-stranded DNA to separate (“denature”) into single strands, making the genetic code accessible for the rest of the process.
- Annealing: The temperature is then sharply reduced, often to 50-65°C, prompting short, custom-designed DNA primers to bind or “anneal” to their specific positions flanking the DNA region of interest. These primers act as starting points for building new DNA strands.
- Extension: Finally, the temperature is readjusted to an optimal range, usually around 72°C, ideal for the activity of a special DNA polymerase enzyme. This enzyme extends from each primer, synthesizing new DNA by adding nucleotides, thus creating a complementary strand to the template.
Through 20 to 40 cycles of these three steps, one original DNA fragment can be amplified into billions of identical copies within a few hours. Because of this amplification, scientists can use PCR to analyze DNA even in samples where genetic material is extremely scarce, such as ancient remains, minor blood stains, or early-stage viral infections.
Applications of PCR
The remarkable versatility of PCR ensures its place as a cornerstone in numerous scientific, clinical, and industrial domains. Its adaptability allows for both basic research and high-stakes decision-making in real-world applications:
- Medical Diagnostics: In healthcare, PCR’s ability to detect even tiny amounts of pathogen nucleic acids has transformed infectious disease management. During the COVID-19 pandemic, PCR testing emerged as the definitive standard due to its unmatched sensitivity and specificity in identifying viral RNA, enabling early isolation and tailored treatment decisions. Beyond COVID-19, PCR helps diagnose many diseases, from HIV and tuberculosis to genetic disorders and cancer.
- Forensic Science: In law enforcement and criminal justice, PCR has made it possible to analyze trace DNA left behind at crime scenes, even from degraded or aged evidence. This revolutionizes criminal investigations, allowing for accurate identification of suspects, exonerating the innocent, and resolving cold cases through familial and paternity testing. PCR’s precision grants forensic analysts a powerful toolkit for delivering justice with scientific certainty.
- Genetic Research: Scientists harness PCR for critical experimental tasks like gene cloning, mutation detection, sequencing, and the study of gene expression. In agriculture, PCR supports crop improvement and the identification of disease resistance genes, ultimately aiding in food security efforts. Evolutionary biology enables the reconstruction of genetic lineages and ancestry, facilitating the study of biodiversity and conservation.
Overall, PCR underpins both fundamental discoveries and real-world interventions, fostering breakthroughs that would be unattainable without its sensitivity and adaptability.
Advancements in PCR Technology
Since its creation, PCR has continued to evolve. New developments have focused on increasing accuracy, speed, and simplicity, driving adoption in new settings and maximizing impact:
- Real-Time PCR (qPCR): This advancement incorporates fluorescent markers, allowing entire reactions to be monitored as they unfold. By delivering qualitative (is the DNA present?) and quantitative (how much DNA is present?) information in real time, qPCR has become a staple for clinical diagnostics, gene expression studies, and drug development. For example, quantifying viral load in patients now guides therapy choices and public health decisions.
- Digital PCR (dPCR): dPCR goes further by splitting the sample into thousands of micro-reactions, enabling the direct, digital counting of DNA molecules. This increases precision and sensitivity, making dPCR indispensable for applications like detecting rare cancer mutations, monitoring minimal residual disease, and conducting prenatal genetic testing with minimal risk to the fetus.
- Portable PCR Devices: The miniaturization of PCR instruments has recently facilitated rapid, point-of-care testing in the field or at the bedside. Portable PCR units now empower frontline workers to tackle outbreaks in remote regions, veterinary staff to monitor animal health, and food inspectors to screen produce for contamination on-site.
Challenges and Considerations
Despite its transformative potential, successful PCR analysis requires careful attention to methodological challenges and technological constraints:
- Contamination Risk: While PCR’s extreme sensitivity is its most significant advantage, it is also susceptible to contamination from ambient DNA, leading to false signals or misleading results. Laboratories must adhere to strict protocols, including using separate workspaces for reagent preparation, sample processing, and frequent decontamination procedures.
- Primer Design: The accuracy and specificity of PCR reactions depend heavily on the design of the primers. Poorly chosen primers may bind to unintended DNA sequences, resulting in non-specific amplification and compromised data reliability. This highlights the necessity for bioinformatics tools and expert insight in crafting optimal primer sets for each application.
- Cost and Accessibility: While basic PCR machines have become more widespread, advanced methodologies such as qPCR and dPCR require significant investment in equipment and consumables. This financial barrier presents challenges for under-resourced laboratories, clinics, and countries, making equitable access to these technologies an ongoing concern for the global scientific and healthcare communities.
Addressing these challenges through continual technological innovations and working to expand access will ensure that PCR’s benefits are more evenly distributed worldwide.
Conclusion
PCR is fundamental to biology, clinical diagnostics, forensics, and genetic research. Its unrivaled ability to amplify even the tiniest fragments of DNA has transformed our understanding of life at the molecular level and fundamentally reshaped how we detect, diagnose, and treat disease. The technology’s evolution ensures that PCR will continue to expand its reach and capability, empowering ever more refined scientific discoveries and breakthroughs in public health for years to come. As we look to the future, PCR’s story remains one of adaptation, accessibility, and profound scientific impact.