According to the 2024 global cancer statistics, nearly 20 million new cancer cases and 9.7 million cancer-related deaths were reported in 2022, highlighting the urgent need for improved therapeutic strategies. While chemotherapy continues to be a mainstay of treatment due to its strong anti-tumor effects, it faces major drawbacks such as poor specificity, toxicity, and drug resistance.
These challenges have driven the search for therapies that combine precision with efficacy. Monoclonal antibodies (mAbs) have shown great promise for their high target specificity, but their limited cytotoxicity and potential for resistance restrict effectiveness. To overcome these issues, antibodies–drug conjugates (ADCs) have emerged, combining the targeting ability of mAbs with the potency of cytotoxic drugs through specialized linkers, to deliver precise, effective, and safer cancer treatments. ADCs are cutting-edge biopharmaceuticals that couple highly specific mAbs to potent cytotoxic agents via chemical linkers.
However, clinical success doesn’t come easy. The journey from concept to approved therapy requires careful consideration of multiple design elements.
Biopharma companies that rely on specialized antibody drug conjugate services understand that each design choice, antibody selection, linker chemistry, payload type, and dosing strategy, can significantly influence safety, efficacy, and patient outcomes.
1. Antibody Selection
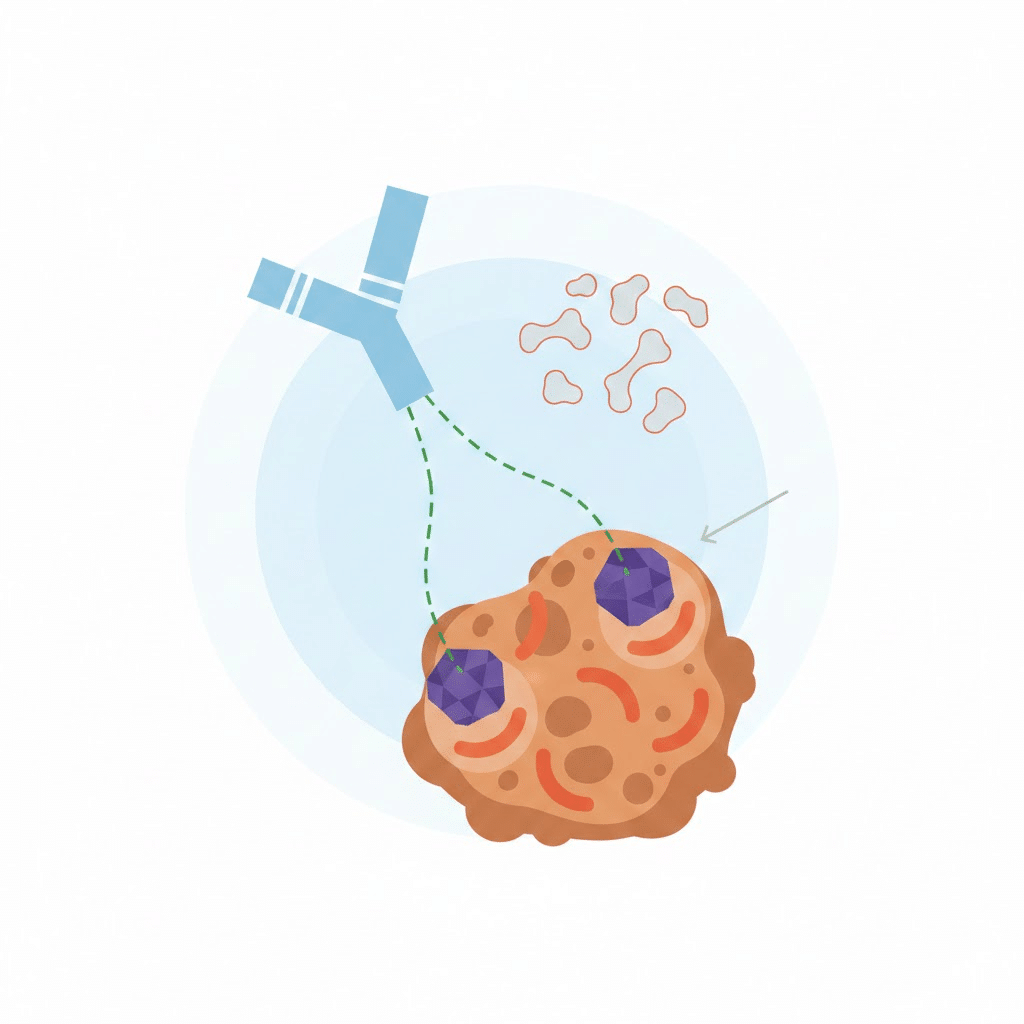
At the core of an ADC is the monoclonal antibody. Its role is to specifically recognize and bind to antigens expressed on cancer cells.
- Specificity: The ideal antibody binds only to tumor-associated antigens, minimizing off-target effects.
- Internalization: Effective ADCs require antibodies that internalize rapidly once bound, ensuring the payload is delivered inside cancer cells.
- Stability: Antibodies must maintain structural integrity during manufacturing, circulation, and delivery.
A poor antibody choice can lead to reduced efficacy or increased toxicity.
2. Linker Chemistry
The linker bridges the antibody and drug payload. While it may seem like a small component, it plays a major role in ADC performance.
- Stable in circulation: The linker must remain intact in the bloodstream to avoid premature release of the toxin.
- Releasable at target site: It should release the payload efficiently once inside tumor cells, often triggered by pH or enzymatic activity.
- Cleavable vs. non-cleavable: Cleavable linkers ensure rapid release, while non-cleavable linkers provide prolonged activity.
The balance between stability and release is critical for safety and therapeutic effect.
3. Payload Selection
The cytotoxic drug attached to the antibody is responsible for killing cancer cells. Payload selection influences potency and tolerability.
- Potency: Since only a limited number of drug molecules can be delivered per antibody, payloads must be highly potent.
- Mechanism of action: Common payloads disrupt microtubules or damage DNA, leading to apoptosis.
- Safety profile: The chosen drug must strike a balance between efficacy against cancer cells and tolerability for patients.
Misaligned payload choices can lead to limited benefit or severe adverse effects.
4. Drug-to-Antibody Ratio (DAR)
The number of payload molecules attached per antibody determines therapeutic strength.
- High DAR: May increase potency but risk aggregation and toxicity.
- Low DAR: Ensures stability but might reduce efficacy.
Optimizing DAR is a delicate process, as both extremes can compromise clinical outcomes.
5. Pharmacokinetics and Dosing
ADCs must remain long enough in circulation to reach tumors while minimizing systemic exposure. Factors like half-life, clearance rate, and biodistribution play a role.
Personalized dosing strategies, sometimes guided by biomarker studies, are increasingly important to balance safety and efficacy across diverse patient populations.
6. Manufacturing and Quality Control
ADCs are complex biologics requiring advanced manufacturing capabilities. Each step, antibody engineering, conjugation, purification, and formulation, must meet stringent quality standards. Therefore, partnering with an experienced CRDMO can streamline this process, ensuring seamless integration from discovery through scale-up and clinical production. A specialized partner manufacturer brings together multidisciplinary expertise in biologics manufacturing, analytical testing, and process optimization, helping biopharma companies accelerate timelines while maintaining consistency, safety, and regulatory compliance. This end-to-end support is essential to translate promising ADC candidates into reliable, commercially viable therapies.
7. Patient-Centric Considerations
Ultimately, design decisions must focus on patient outcomes:
- Efficacy in heterogeneous tumors: ADCs should address varying antigen expression levels.
- Reduced toxicity: By targeting only cancer cells, side effects like neuropathy or cytopenias should be minimized.
- Combination potential: ADCs are increasingly studied alongside immunotherapies and checkpoint inhibitors, expanding their clinical utility.
8. Emerging Innovations in ADC Design
Beyond the established design elements, next-generation ADCs are exploring new frontiers. Advances include the use of bispecific antibodies that can target multiple antigens simultaneously, improving precision against heterogeneous tumors. Novel payload classes, such as immune-modulating drugs, are being tested to expand therapeutic applications beyond traditional cytotoxics. Additionally, site-specific conjugation technologies are enhancing the stability and consistency of ADCs, reducing variability in drug-to-antibody ratios. These innovations highlight how rapidly the field is evolving.
Final Thoughts
The promise of antibody-drug conjugates lies not just in their novel mechanism but in the precision of their design. Every decision, from antibody specificity and linker chemistry to payload potency and dosing, affects clinical performance.
When done right, ADCs can deliver targeted, effective therapies that improve survival rates and quality of life for patients battling cancer.
As oncology research moves forward, ADCs stand as a shining example of how science, engineering, and patient focus intersect to transform modern medicine.