Adavosertib (MK-1775) Wee1 inhibitor is one of the pioneering developments in targeted therapy of cancer. On one hand, with the ongoing development of precision medicine, cell cycle regulators have become a promising drug target to researchers.
Wee1 kinase is a key checkpoint protein that regulates cell cycle development that is particularly important in the preservation of genomic integrity. Adavosertib suppresses DNA repair by Wee1, an enzyme that mediates the repair of cancer cell DNA damage, inducing apoptosis.
The Adavosertib (MK-1775) Wee1 inhibitor has become a powerful mediator when used together with the DNA-damaging agents to overcome resistance and exclusively to target tumor cells that have defective p53 signaling. Click on this link for more.
Wee1 and the Cell Cycle
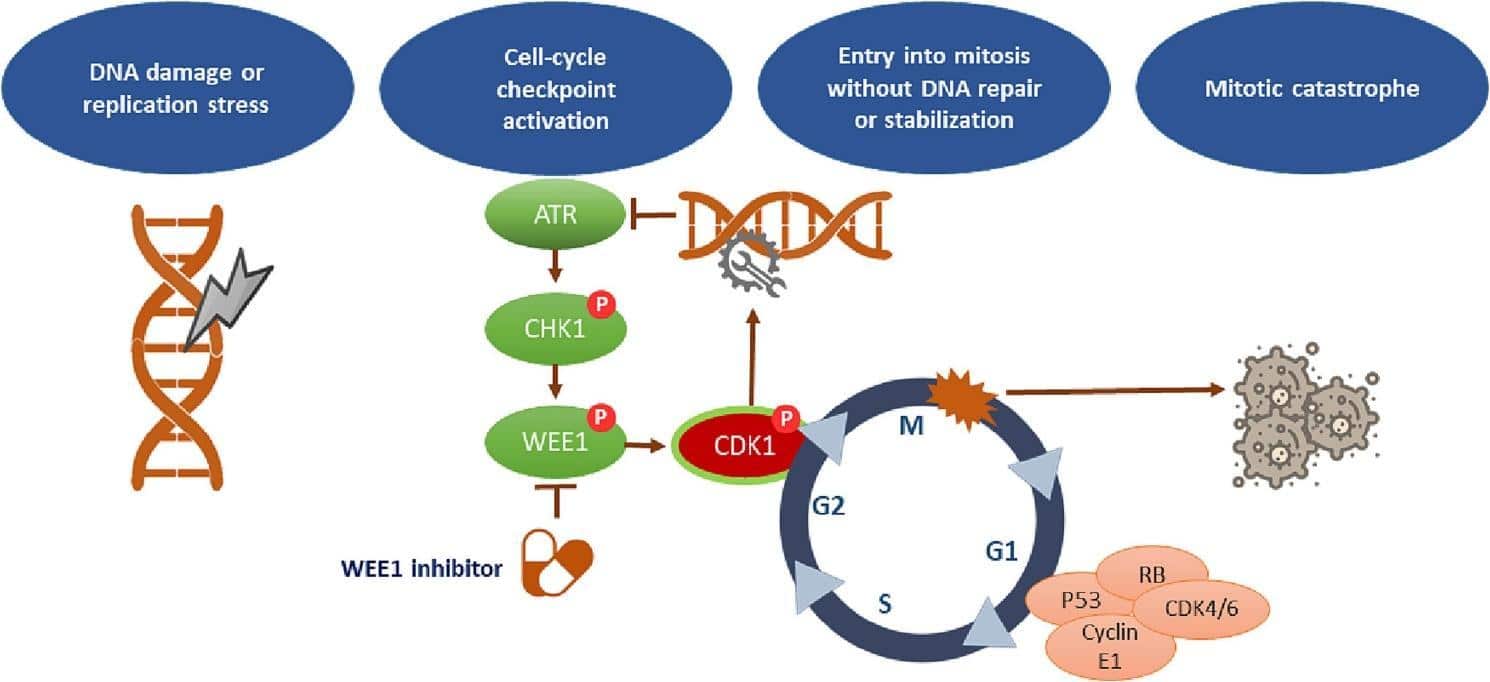
Wee1 is a serine/threonine kinase that plays a major role as the regulator of G2/M checkpoint. In usual conditions, Wee1 phosphorylates and suppresses cyclin-dependent kinase 1 (CDK1) to block an untimely transition into mitosis. This is a regulatory measure so that the cells can repair the DNA damage in the sufficient time before division.
In most types of cancers, however, defects in tumor suppressor genes, especially TP53, undermine the G1 checkpoint, leaving cells under the control of the G2/M checkpoint to repair DNA damage. This dependency is used in the Adavosertib (MK-1775) Wee1 inhibitor. Adavosertib prevents Wee1, which is the last obstacle between damaged cells going into mitosis, resulting in the mitotic catastrophe and cell death.
This principle of synthetic lethality allows the Adavosertib (MK-1775) Wee1 inhibitor to be particularly effective against p53-deficient tumors which are unable to arrest at G1 and which are largely dependent on the control of Wee1.
How It Works
Adavosertib (MK-1775) is a Wee1 inhibitor that blocks the catalytic activity of Wee1 kinase by binding specifically to its ATP-binding region. Consequently, CDK1 stays active and promotes premature mitotic entry even when DNA damage is present.
Chromosomal instability and apoptotic signals result from this forced progression, which overwhelms the cell’s repair mechanism. By blocking tumor cells’ ability to repair DNA damage caused by DNA-damaging drugs like cisplatin, gemcitabine, or radiation therapy, the Adavosertib (MK-1775) Wee1 inhibitor is most effective when administered in combination with these other treatments.
As a chemosensitizer, Adavosertib has been proven in preclinical research to increase tumor sensitivity to radiation and chemotherapy in a variety of tumor types.
Pharmacological Properties
A tiny molecule with excellent oral bioavailability and pharmacokinetics, Adavosertib (MK-1775) inhibits Wee1. After an oral dose, it reaches its target efficiently, distributes throughout the body, and inhibits Wee1 for an extended period of time. It helps keep side effects to a minimum in clinical studies by selectively targeting Wee1 rather than similar kinases.
To minimize side effects while maximizing therapeutic effect, adavosertib is usually taken at intervals throughout chemotherapy regimens. Dosage techniques and combinations are being fine-tuned through ongoing research to maximize therapeutic outcomes.
Clinical Applications
The Wee1 inhibitor Adavosertib (MK-1775) has demonstrated encouraging results in the treatment of certain cancers, especially those including TP53 mutations. Its potential application in treating malignancies of the ovary, lung, colon, and pancreas has been investigated in preliminary clinical studies.
Compared to chemotherapy alone, carboplatin and paclitaxel combination therapy improves progression-free survival in ovarian cancer. Wee1 inhibition speeds up the buildup of unrepaired DNA damage, which is responsible for the synergistic impact.
One potential solution to the huge problem of platinum resistance in NSCLC is the Adavosertib (MK-1775) Wee1 inhibitor, which increases the efficacy of DNA-damaging regimens. Head and neck squamous cell carcinoma has also shown improvement with combination therapy, leading to higher rates of tumor regression.
Additionally, in cancers lacking homologous recombination, clinical trials have shown that Adavosertib may increase the DNA repair stress and hence the effects of PARP inhibitors. Wee1 inhibitor Adavosertib (MK-1775) is now in a prime position to be a cornerstone of combination therapy for malignancies driven by DNA damage.
Ensuring Safety and Tolerance
Wee1 inhibitor Adavosertib (MK-1775) is often well-tolerated. Fatigue, nausea, diarrhea, and hematologic toxicities such neutropenia and thrombocytopenia are the most often reported side effects in clinical trials. Because cells in quickly dividing tissues, such as bone marrow and mucosal tissues, are more vulnerable to cell cycle interruption, these effects are in line with its mechanism.
Mitigating adverse effects requires careful dosing schedule in addition to supportive care. It is important to note that toxicities can usually be reversed by stopping the treatment or reducing the dosage. The ideal compromise between efficacy and tolerance will be defined through ongoing investigation.
Methods for Resistance
Prolonged use of the Adavosertib (MK-1775) Wee1 inhibitor can lead to resistance, as is the case with many targeted medicines. Possible explanations include adaptive reprogramming of cell cycle regulators, enhanced DNA repair capability, or overexpression of compensating checkpoints such Chk1.
In order to address these side effects, scientists are looking into dual inhibition techniques. These involve combining Adavosertib with other drugs that block DNA damage response pathways, such as ATR inhibitors, Chk1 inhibitors, or PARP inhibitors. Adavosertib (MK-1775), a Wee1 inhibitor, has shown promising early results when used in combination with other drugs, demonstrating its versatility and potential as a basis for rational drug design.
Looking Ahead
Precision oncology is where the Adavosertib (MK-1775) Wee1 inhibitor will be used in the future. The development of genomic profiling has opened the door to more targeted and efficient therapy options for individuals whose tumors display p53 mutations, DNA repair defects, or Wee1 overexpression.
In addition, biomarker-driven trials are being investigated by researchers to identify the molecular signals that might forecast the most beneficial outcomes. Further expansion of Adavosertib’s therapeutic utility may be possible by its combination with other novel modalities, such as immunotherapy and radiation.
Adavosertib (MK-1775) was the first Wee1 inhibitor to show therapeutic promise; developing next-generation inhibitors has the potential to increase potency, selectivity, and safety.
The Adavosertib (MK-1775) Wee1 inhibitor is taking precision medicine one step closer to its ultimate aim of focused, effective, and long-lasting cancer therapies as research shows how strategically inhibiting checkpoint kinases can tip the scales in favor of cancer eradication.